

產(chǎn)品中心


Overview
-
 Western Blot analysis using His-tag Polyclonal Antibody against HEK293 cells transfected with vector overexpressing His tag (1) and untransfected (2). Antibody was diluted at 1:2000. Secondary antibody(catalog#:RS0002) was diluted at 1:20000
Western Blot analysis using His-tag Polyclonal Antibody against HEK293 cells transfected with vector overexpressing His tag (1) and untransfected (2). Antibody was diluted at 1:2000. Secondary antibody(catalog#:RS0002) was diluted at 1:20000 -
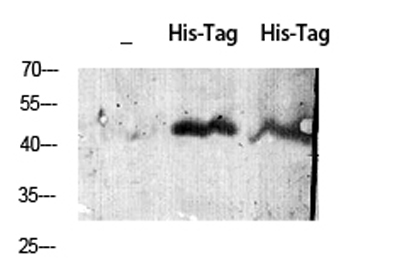
 Western Blot analysis of HIS-protein cells using His-tag Polyclonal Antibody diluted at 1:2000. Secondary antibody(catalog#:RS0002) was diluted at 1:20000
Western Blot analysis of HIS-protein cells using His-tag Polyclonal Antibody diluted at 1:2000. Secondary antibody(catalog#:RS0002) was diluted at 1:20000
關(guān)閉
在線咨詢
Online consultation
-
在線咨詢
-
技術(shù)支持

關(guān)注微信公眾號

 下載說明 ①
下載說明 ①



